8.4. A SHORT APPLICATION TO CHEMISTRY 159
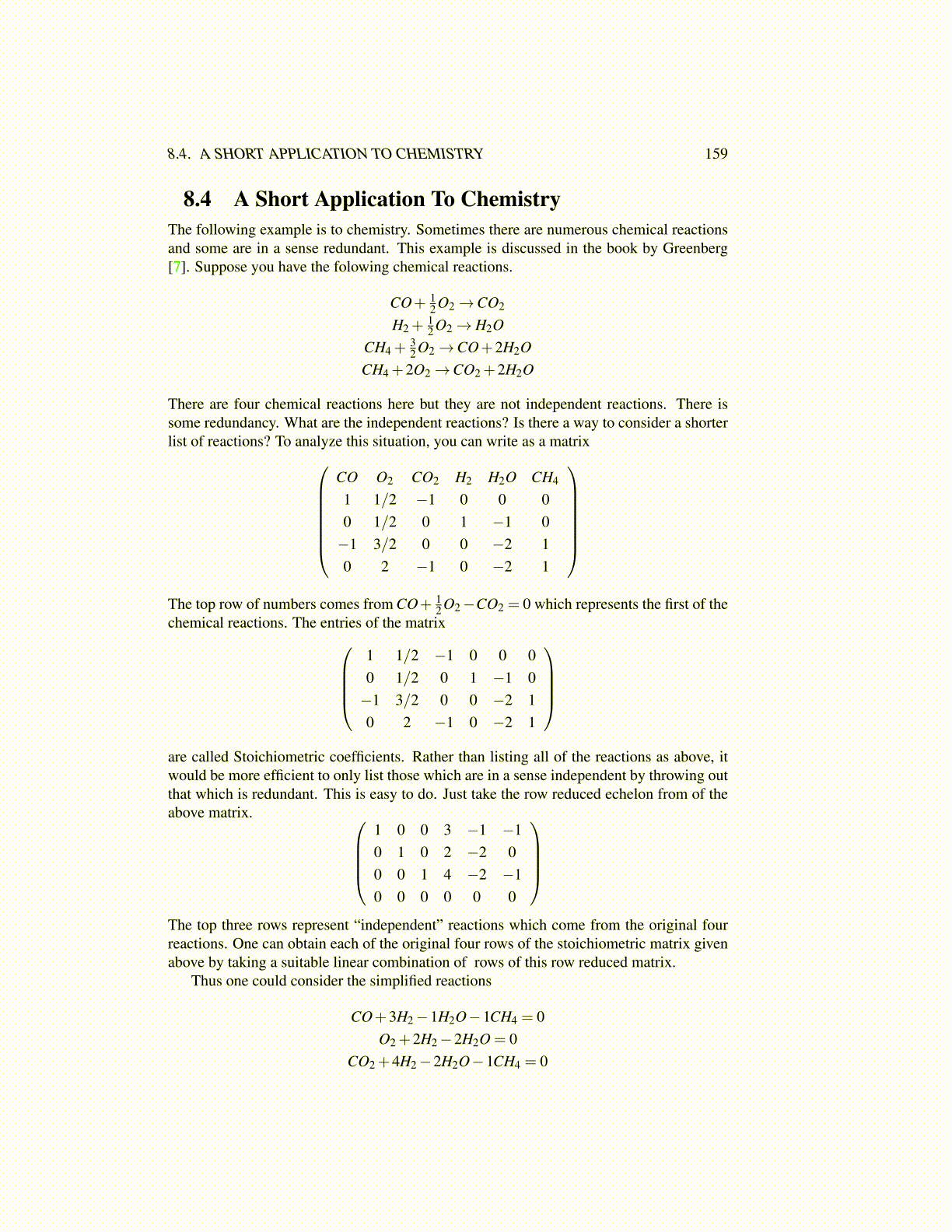
8.4 A Short Application To ChemistryThe following example is to chemistry. Sometimes there are numerous chemical reactionsand some are in a sense redundant. This example is discussed in the book by Greenberg[7]. Suppose you have the folowing chemical reactions.
CO+ 12 O2→CO2
H2 +12 O2→ H2O
CH4 +32 O2→CO+2H2O
CH4 +2O2→CO2 +2H2O
There are four chemical reactions here but they are not independent reactions. There issome redundancy. What are the independent reactions? Is there a way to consider a shorterlist of reactions? To analyze this situation, you can write as a matrix
CO O2 CO2 H2 H2O CH4
1 1/2 −1 0 0 00 1/2 0 1 −1 0−1 3/2 0 0 −2 10 2 −1 0 −2 1
The top row of numbers comes from CO+ 1
2 O2−CO2 = 0 which represents the first of thechemical reactions. The entries of the matrix
1 1/2 −1 0 0 00 1/2 0 1 −1 0−1 3/2 0 0 −2 10 2 −1 0 −2 1
are called Stoichiometric coefficients. Rather than listing all of the reactions as above, itwould be more efficient to only list those which are in a sense independent by throwing outthat which is redundant. This is easy to do. Just take the row reduced echelon from of theabove matrix.
1 0 0 3 −1 −10 1 0 2 −2 00 0 1 4 −2 −10 0 0 0 0 0
The top three rows represent “independent” reactions which come from the original fourreactions. One can obtain each of the original four rows of the stoichiometric matrix givenabove by taking a suitable linear combination of rows of this row reduced matrix.
Thus one could consider the simplified reactions
CO+3H2−1H2O−1CH4 = 0O2 +2H2−2H2O = 0
CO2 +4H2−2H2O−1CH4 = 0